- Systematic review
- Open access
- Published:
Strategies for monitoring and updating clinical practice guidelines: a systematic review
Implementation Science volume 7, Article number: 109 (2012)
Abstract
Background
Scientific knowledge is in constant change. The flow of new information requires a frequent re-evaluation of the available research results. Clinical practice guidelines (CPGs) are not exempted from this phenomenon and need to be kept updated to maintain the validity of their recommendations. The objective of our review is to systematically identify, describe and assess strategies for monitoring and updating CPGs.
Study design and setting
We conducted a systematic review of studies evaluating one or more methods of updating (with or without monitoring) CPGs or recommendations. We searched MEDLINE (PubMed) and The Cochrane Methodology Register (The Cochrane Library) from 1966 to June 2012. Additionally, we hand-searched reference lists of the included studies and the Guidelines International Network book of abstracts. If necessary, we contacted study authors to obtain additional information.
Results
We included a total of eight studies. Four evaluated if CPGs were out of date, three updated CPGs, and one continuously monitored and updated CPGs. The most detailed reported phase of the process was the identification of new evidence. As opposed to studies updating guidelines, studies evaluating if CPGs were out of date applied restricted searches. Only one study compared a restricted versus an exhaustive search suggesting that a restricted search is sufficient to assess recommendations’ Validity. One study analyzed the survival time of CPGs and suggested that these should be reassessed every three years.
Conclusions
There is limited evidence about the optimal strategies for monitoring and updating clinical practice guidelines. A restricted search is likely to be sufficient to monitor new evidence and assess the need to update, however, more information is needed about the timing and type of search. Only the exhaustive search strategy has been assessed for the update of CPGs. The development and evaluation of more efficient strategies is needed to improve the timeliness and reduce the burden of maintaining the validity of CPGs.
Background
Scientific knowledge is in constant change, and new information requires frequent assessment to determine whether it changes the knowledge base [1]. A clinical practice guideline (CPG) may be considered out of date if it does not include all recent, valid, and relevant evidence or does not reflect current clinicians’ experience and patients’ values and preferences [2]. CPGs, hence, need to be updated regularly to remain valid.
Shekelle et al. evaluated the validity of a cohort of CPGs [3]. Survival analysis indicated that 90% of CPGs were still valid in 3.6 years, but 50% were out of date in 5.8 years [3]. Based on these results, most methodological handbooks for the development of CPGs propose three years as a reasonable time frame to update their guidelines [1, 4].
In 2007, Moher et al. conducted a study about when and how to update systematic reviews [5]. Although not included in the objectives, the authors identified and described several methods for updating CPGs. In their conclusions the authors argue that the methodology for updating CPGs, as opposed to systematic reviews, is well established. Nevertheless, a recent international survey showed high variability and a lack of standardization in the CPGs updating processes [6].
A few studies have evaluated different strategies for the CPGs updating process [3, 7, 8], however, no systematic reviews have been conducted about this topic. We therefore undertook a systematic review of the studies that assessed strategies for monitoring and updating CPGs.
Methods
Information sources and search
We performed a search in June 2012 in MEDLINE (accessed through PubMed, from 1966 onwards) and The Cochrane Methodology Register (accessed through The Cochrane Library, Issue 6 2012). We included studies regardless of their language or publication status. The search strategy is available as supplementary data (Additional file 1). Additionally, we hand searched reference lists of the included studies and in the Guidelines International Network book of abstracts (available online from 2009 until 2011 in http://www.g-i-n.net/). If necessary we contacted study authors to obtain additional information. Two authors were in charge of performing all searches (IS, LMG).
Eligibility criteria
-
1.
Type of study: We included studies evaluating one or more methods of updating (with or without monitoring) evidence-based CPGs or recommendations. We excluded studies that only reported updating methods (without actually testing them), methodological handbooks or CPGs updates. We made no restriction by health topic.
-
2.
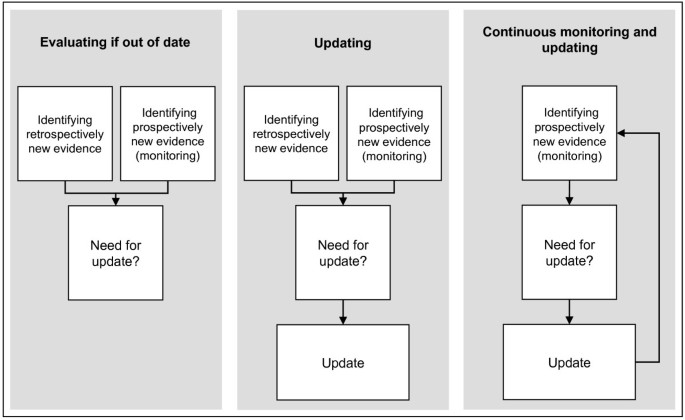
Type of design: We included studies assessing strategies for evaluating if CPGs are out of date; for updating CPGs; for continuous monitoring and updating of CPGs (Figure 1).
Study selection
Two authors independently reviewed the titles and abstracts, as well as the full text of the selected articles for a more detailed evaluation and approved their final inclusion (LMG, IAR). Any disagreement among the authors was initially resolved by consensus, and if necessary, we consulted a third author (IS).
Data extraction strategy
Two authors independently extracted information from the included studies using an ad hoc form (LMG, IAR) that can be requested from the authors. Disagreements among the authors were resolved by consensus and, if required, by a third author (IS). We contacted study authors by email when more information was needed.
We extracted the following information from each study: institution or guideline program and country; objective and design of the study; sample (selection and size) and health topic; time to update (number of years since the development of the original CPG); stages of the strategies; type of search (restricted or exhaustive, classified depending on databases consulted and types of studies searched); resource use (number of participants and time spent); search and update results; and advantages and limitations of strategies reported by the authors.
Data synthesis and presentation
We describe included studies both individually and narratively. We calculated the search performance of the strategies (as a proportion of included documents from all documents identified); and update performance of the strategies (as a proportion of updated recommendations or CPGs from all evaluated recommendations or CPGs).
Results
Study selection
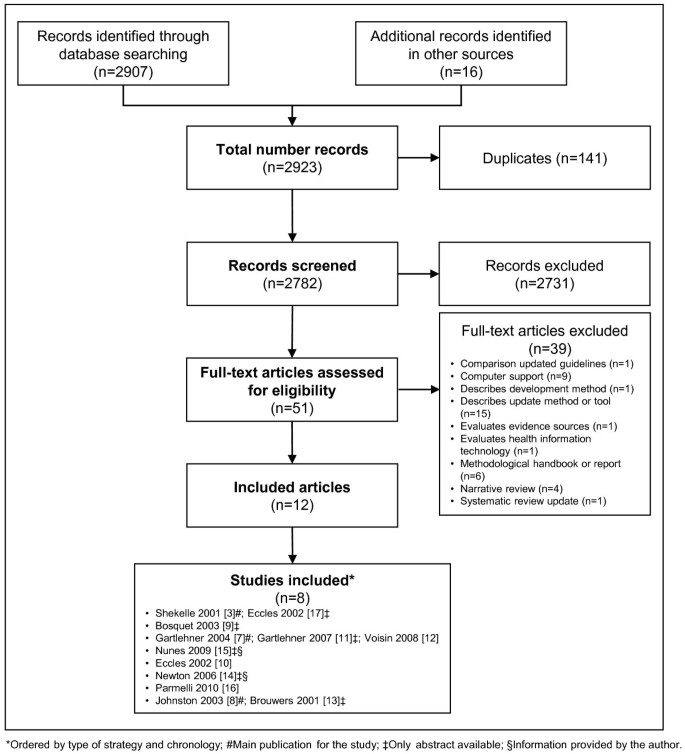
The screening process is summarized in the flow diagram (Figure 2). We initially identified 2,923 references from our search strategy, and excluded 141 duplicates and 2,731 references after examining the title and abstract. We reviewed 51 full texts and we excluded 39 references (Additional file 2). We finally included eight studies corresponding to 12 original references [3, 7–17]. Three studies were only available as abstracts [9, 14, 15]. We successfully contacted two of the first abstracts’ authors to obtain additional information of these studies [14, 15].
Study characteristics
Four studies assessed strategies evaluating if CPGs were out of date [3, 7, 9, 15], three studies assessed strategies for updating CPGs [10, 14, 16], and one study assessed a strategy for continuous monitoring and updating CPGs [8] (Table 1). Five studies assessed a single strategy for: evaluating if CPGs were out of date [3, 9, 15], for updating CPGs [14] or for continuous monitoring and updating of CPGs [8]; three studies compared two strategies: two different strategies for evaluating if CPGs were out of date [7] or ‘de novo’ development versus updating strategies [10, 16]. Most of the studies evaluated full CPGs (range from one to 20 CPGs) [3, 8–10, 14, 15], however none of them included a random sample. The topics included varied widely, with cancer being the most frequent [8, 9, 16]. The time to update source documents ranged from one to eight years. The common stages identified in the strategies were: assessment of need to update; scope definition of the update; working group composition; search strategy; selection, assessment, and synthesis of the evidence; recommendations update (only for updating and continuous monitoring); and presentation format of the updates (for strategies for updating and continuous monitoring) (Table 2).
Strategies for evaluating if CPGs are out of date
Shekelle et al. [3] developed a strategy to assess the validity of CPGs based on identified new evidence through restricted searches (reviews, editorials, or commentaries in general or specialised journals) and through a survey of clinical experts (Table 2). The CPGs were classified by the type of update required as: major update—new evidence suggests the need for new recommendations; minor update—new evidence supports changes to recommendations or refinement of existing recommendations; and the recommendations remain valid. The participants in this updating process were two methodologists and 146 clinical experts. The clinical experts participated in a survey to assess current validity of the guideline recommendations and to identify new evidence (71% of response rate). The search performance was 2.9% (208 articles reviewed from 7,150 articles initially identified) (Table 3). The authors concluded that 76.5% of the guidelines needed to be updated (13/17 CPGs) (Table 3). The results of survival analysis indicated that 90% of CPGs were still valid at 3.6 years (95% confidence interval (CI) 2.6 to 4.6 years), but 50% of CPGs were out of date at 5.8 years (95% CI 5.0 to 6.6 years). The main reported limitation of the study included no previous validation of the method of assessing obsolescence.
Bosquet et al. [9] monitored the literature to assess the need for updating a CPG (Table 2). This approach applied an exhaustive search and evaluated the impact of new studies on existing guidelines (consistent, inconsistent or concern new topics) by consultation with the original working group (mail and meetings). The search performance was 45.2% (118 references submitted to the working group from 261 references initially identified) (Table 3).
Gartlehner et al. [7] compared two search strategies to identify new evidence for assessing the need to update six topics of one CPG: a modified Shekelle et al. [3] search versus an exhaustive search (Table 2). The researchers modified in three consecutive phases the literature search developed by Shekelle et al. [3], mainly by eliminating some of the databases (omitting a general web site search and confining the MEDLINE search to Abridged Index Medicus journals). The participants were 10 methodologists (five for each model) and 13 clinical experts. The clinical experts participated in a survey similar to the one carried out in Shekelle et al. [3] (28% response rate). The search performance was 2.6% for the modified Shekelle et al. [3] search (36 eligible studies from 1,382 citations initially identified) and 1.2% for the exhaustive search (45 eligible studies from 3,687 citations initially identified) (Table 3). The reported strength of the modified Shekelle et al. [3] method was that it offered a search strategy that detected fewer citations to review and identified all studies that triggered an update. However, the study only included prevention topics; the comparison of the number of abstracts and full texts between the two approaches was limited because the Shekelle et al. [3] search was an integral part of the exhaustive search; and the experts who assessed the importance of the studies not identified were not blinded to the type of search approach.
Nunes et al. [15] described a restricted search and a review of new CPGs to decide whether their guideline required an update (Table 2). The participants were three methodologists and two experts. The search performance was 28% (seven guidelines reviewed from 25 guidelines initially identified) (Table 3). No recommendations needed to be updated because identified recommendations in other CPGs were consistent with the existing CPG (Table 3). Less time was required to perform the update strategy than the time estimated to perform an exhaustive search. The need to judge the applicability of recommendations from other guidelines was one of the difficulties reported.
Strategies for updating CPGs
Eccles et al. [10] compared the updating process with the original development process of two CPGs (Table 2). In both the development and updating process new evidence was identified by exhaustive search. Recommendations were classified as: new (if new evidence was identified); refined (if supplementary evidence was identified); and unchanged (if no new evidence was identified). Two methodologists and 14 clinical experts participated. The search performance was 1.0% for each guideline (e.g., for angina there were 59 acceptable papers from 5,941 citations initially identified) (Table 3). Recommendations remained unchanged (Table 3). Among the reported strengths were that some members also had participated in the original development of the guidelines and were better trained and were more familiar with the literature. These members also identified other relevant evidence. The main reported limitation of the updating strategy was that it was as burdensome, time consuming, and costly.
Newton et al. [14] updated an existing CPG (by updating and expanding the original search) and adapted it to the local context (by adding research questions for the local setting) (Table 2). The participants were nine methodologists and six experts. The search performance was 0.2% (43 studies included from 19,423 citations initially identified) (Table 3). The authors spent nine months to update 11 research questions and to develop seven new research questions (Table 3). The authors acknowledged that they were uncertain whether the process was more time efficient due to the additional search needed to cover local adaptation issues.
Parmelli et al. [16], similarly to Eccles et al. [10], compared an updating method with the original development process in a set of 15 recommendations (Table 2). The GRADE framework was used in both developing and updating recommendations [18]. For the updating process, new exhaustive searches were designed. The number of participants was not specified, but they described the main characteristics of its coordinator, methodological, and expert group. The researchers spent eight months to update 15 recommendations. The search performance was 3.5% (24 papers included from 686 records initially screened) (Table 3). Forty per cent of the recommendations (6/15) were completely updated due to identification of new evidence, leading to a change in recommendation classification or to redefinition of the original clinical questions (Table 3). The advantages of this updating strategy, as reported by the authors, were better collaboration among the members of the updating group, and improved methodology, leading to more relevant recommendations for clinical practice. On the other hand, the updating process was neither time nor resource saving.
Strategies for continuous monitoring and updating of CPGs
Johnston et al. [8] carried out a pilot study of a monitoring strategy in 20 CPGs (Table 2). The strategy included four steps: continuous and exhaustive searching; reviewing the new evidence; revising recommendations; and announcing new evidence and modified recommendations. The researchers used the term ‘living’ practice guideline, defined as ‘integrated evidence update into the original report.’ The information about the participants was limited. The researchers applied this strategy during a year. The search performance was 23.75% (19 citations with impact on recommendations from 80 citations initially identified) (Table 3). In 30% (6/20) of the guidelines, a change in their clinical recommendations was made (Table 3). The reported advantages of this method were that often the experts identified new evidence before it was available in electronic databases, and they therefore proposed that the literature search could be done quarterly and limited to Medline, the Cochrane library, and meeting proceedings. The authors described the process as intensive, and they foresaw that the updating process would need to change if many more guidelines had to be updated.
Discussion
Our systematic review shows that the available research about the monitoring and updating of CPGs is scarce, and little is known at present about the most efficient way of keeping guidelines valid. Furthermore, the suboptimal reporting and the wide variability in the design, choice of outcomes, type of search strategy, and breadth of topics makes the assessment of the included studies difficult.
We identified eight studies with three main different objectives: evaluation if guidelines are out of date and, hence, how often they need updating [3, 7, 9, 15], and the evaluation of updating strategies without [10, 14, 16] or with continuous monitoring new evidence [8]. Regardless of the goal, the included studies followed similar stages in their process. However, authors generally did not describe the process in enough detail to clearly identify the different stages.
The most detailed phase of the studies was the literature search method. Strategies evaluating if CPGs were out of date [3, 7] used more restricted searches (limited to reviews, editorials or commentaries of specific journals and expert collaboration) than updating strategies. These approaches aim to identify the relevant new evidence without performing an exhaustive search; however, they risk omitting key references (references that trigger a modification of a recommendation). The evidence for their performance is so far limited [3, 7], with only one single head-to-head comparison available between a restricted search and an exhaustive search [7]. According to the results observed in Gartlehner et al. [7], the evidence identified by this more restricted search would be enough to assess the need to update a CPG. However, it remains unclear whether these search results would be a sufficient way to actually update recommendations.
Strategies that aimed to update [10, 16] or continuously monitor and update CPGs [8] used more exhaustive searches and were generally very similar to the ones used in the development processes. These exhaustive search strategies are more time-consuming than restricted searches as the searches trade what is hoped will be higher sensitivity for low specificity. However, the study of Gartlehner et al. [7] suggests that sensitivity for key studies is not increased by an exhaustive search process.
Although search strategies were specified in the included studies, search results were difficult to compare across studies due to the variability of the search performance outcomes (e.g., Shekelleet al.[3] reported the articles reviewed and Gartlehner et al. [7] reported just the eligible studies). Furthermore, reported results would need to be adjusted by the number of CPGs or recommendations updated and the time to update them. Unfortunately, this information was not available for most of the studies.
Only three studies [3, 8, 16] reported updating performance results. For example, Parmelli et al. updated 40% of the recommendations about breast, colorectal, and lung cancer treatment three years after the development of the recommendations [16]. The update process should focus on the recommendations, instead of the full guideline, because it could provide an opportunity to make the process more efficient. The GRADE system [19], already used by Parmelli et al. [16], could provide a framework to systematically and explicitly assess to what extent the new evidence can modify the different factors that ultimately influence the direction and strength of the recommendations (quality of the evidence, balance between benefits and harms, patients’ values and preferences, and resource use) [18].
Future research studies should standardize outcomes of interest. In relation to the search performance, authors should report the number of key references (references that triggered a modification of a recommendation) from the number of references initially identified. In relation to the updating performance, measures should include the number of recommendations updated from total of the CPG recommendations. Finally, regarding resource use, studies should report the number of participants and the time spent in the updating process.
All the studies described the composition of the updating working group involved in the process; however, the total number of participants and their roles was generally unclear. In general, similarly to the development of guidelines, there was a core method group and a larger group of clinical experts (with a variable degree of involvement). Only three studies included the time devoted to the process (range eight to ten months) [8, 14, 16]. Nevertheless, this information is difficult to generalise because it is highly dependent on the goal of the strategy, the scope of the guideline, the methodological expertise of the group members or the economic resources of the different institutions involved, among other possible factors. Had this information been more detailed, it could have been used as a guide to assist in the development and updating of CPGs programs.
There is a need to develop more efficient monitoring and updating strategies for CPGs and, for this, rigorous research is crucial. The gold standard strategy to update CPGs should include the identification of new evidence by an exhaustive search and the update of the recommendations [10, 16]. One of the most resource-intensive phases where there is more room for improvement is the literature search. Other restricted search strategies or resources like McMaster Premium LiteratUre Service or Clinical Queries, have shown to improve the efficacy of keeping systematic reviews up to date [20]. This remains to be replicated in guidelines. Other areas to explore are the performance of databases (e.g., EMBASE) or the role of clinical experts as a source of references, with these more limited strategies [21].
In relation to survival time, Shekelle et al. [3] proposed, as a general rule, to assess the validity of CPGs every three years. Regardless of the time frame, which is highly dependent on the health topic, it would be desirable to develop a dynamic warning system to identify new relevant evidence (monitoring) and evaluate the need to update. A potential tool that could be easily implemented to monitor the new evidence is the automated periodic searches in MEDLINE, using their Selective Dissemination of Information service [22]. Complementary, guideline groups could monitor publication rate of specific MeSH terms in relation with a CPG topic. At the moment this information is not readily available in biomedical databases (e.g., MEDLINE).
Our study has several strengths. We performed an exhaustive systematic review including contacting authors for additional information. In our review, we proposed a novel classification of CPGs updating strategies according to their objective: evaluation CPGs obsolescence, updating, and continuously monitoring and updating CPGs. Finally, we also propose standardised reporting framework, including outcomes, for research purposes.
Our study has limitations. First, it is possible that we did not identify all studies due to publication bias or to the omission of some more specialised information sources (e.g., conference proceedings). Second, the difficulty of synthesizing, evaluating, and comparing complex methodological studies, without a standardized reporting, as opposed to systematic reviews [23] or comparative effectiveness reviews [24], makes the analysis and interpretation of results challenging.
Conclusions
There is limited evidence about the optimal strategies for monitoring and updating CPGs. A restricted search is likely to be sufficient to monitor new evidence and assess the need to update; however, more information is needed about the timing and type of search. Only the exhaustive search strategy has been assessed for the update of CPGs.
Our review highlights suboptimal reporting, and wide variability in the design, choice of outcomes, and type of search strategy of the available studies. The development and evaluation of more efficient strategies is needed to improve the timeliness and reduce the burden of maintaining the validity of CPGs. Future research studies should adequately report their methods and results.
Abbreviations
- CI:
-
Confidence interval
- CPG:
-
Clinical practice guideline.
References
Working Group on CPG Updates: Updating Clinical Practice Guidelines in the Spanish National Health System: Methodology Handbook. 2009, Madrid: National Plan for the National Health System of the Spanish Ministry for Health and Social Policy; Aragon Health Sciences Institute (I+CS); Clinical Practice Guidelines in the Spanish National Health System: I+CS
Clark E, Donovan EF, Schoettker P: From outdated to updated, keeping clinical guidelines valid. Int J Qual Health Care. 2006, 18 (3): 165-166. 10.1093/intqhc/mzl007.
Shekelle PG, Ortiz E, Rhodes S: Validity of the Agency for Healthcare Research and Quality clinical practice guidelines: how quickly do guidelines become outdated?. JAMA. 2001, 286 (12): 1461-1467. 10.1001/jama.286.12.1461.
National Institute for Health and Clinical Excellence (January 2009): The guidelines manual. 2009, London: National Institute for Health and Clinical Excellence, Available from: http://www.nice.org.uk.
Moher D, Tsertsvadze A, Tricco AC, Eccles M, Grimshaw J, Sampson M: A systematic review identified few methods and strategies describing when and how to update systematic reviews. J Clin Epidemiol. 2007, 60 (11): 1095-1104.
Alonso-Coello P, Martínez García L, Carrasco JM, Solà I, Qureshi S, Burgers JS: The updating of clinical practice guidelines: insights from an international survey. Implement Sci. 2011, 6: 107-10.1186/1748-5908-6-107.
Gartlehner G, West SL, Lohr KN: Assessing the need to update prevention guidelines: a comparison of two methods. Int J Qual Health Care. 2004, 16 (5): 399-406. 10.1093/intqhc/mzh081.
Johnston ME, Brouwers MC, Browman GP: Keeping cancer guidelines current: results of a comprehensive prospective literature monitoring strategy for twenty clinical practice guidelines. Int J Technol Assess Health Care. 2003, 19 (4): 646-655.
Bosquet L Guillo S Gory-Delabaere G Fervers B COSOR: Improving Outcomes Through Health Technology Assessment. 19th Annual Meeting of the International Society of Technology Assessment in Health Care. Technological and scientific literature monitoring for updating clinical practice guidelines: example with use of PET scanning in patients with cancer. 2003, Alberta, Canada: Canmore, 272-
Eccles M, Rousseau N, Freemantle N: Updating evidence-based clinical guidelines. J Health Serv Res Policy. 2002, 7 (2): 98-103. 10.1258/1355819021927746.
Gartlehner G, West SL, Lohr KN: Assessing the need to update prevention guidelines: a comparison of two methods. Evid Base Libr Inform Pract. 2007, 2 (2): 40-41.
Voisin CE, de la Varre C, Whitener L, Gartlehner G: Strategies in assessing the need for updating evidence-based guidelines for six clinical topics: an exploration of two search methodologies. Health Info Libr J. 2008, 25 (3): 198-207. 10.1111/j.1471-1842.2007.00765.x.
Brouwers M, Johnston M, Browman G: 17th Annual Meeting of the International Society of Technology Assessment in Health Care: Building Bridges Between Policy, Providers, Patients and Industry. Results of a prospective study to keep guidelines current. 2001, Philadelphia, Pennsylvania, USA
Newton S, Merlin T, Forbes D, Phelps A, Creamer M, Hiller J: Third Annual Meeting Health Technology Assessment International. Challenges in updating evidence-based clinical practice guidelines. 2006, Adelaide, Australia, 141-
Nunes V, Shaw E: Proceedings of the 6th Guidelines International Network Conference. When to Update Guidelines. A pragmatic approach. 2009, Lisbon
Parmelli E, Papini D, Moja L, Bandieri E, Belfiglio M, Ciccone G: Updating clinical recommendations for breast, colorectal and lung cancer treatments: an opportunity to improve methodology and clinical relevance. Ann Oncol. 2011, 22 (1): 188-194. 10.1093/annonc/mdq324.
Eccles M, Shekelle P, Grimshaw J, Woolf S: Satellite Symposium Clinical Practice Guidelines. Updating of CPGs. Experiences from the U.K. (& USA). The Challenge of Collaboration. 2002, Berlin, Germany: International Society of Technology Assessment in Health Care, 238-
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P: GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008, 336 (7650): 924-926. 10.1136/bmj.39489.470347.AD.
Guyatt GH, Oxman AD, Schünemann HJ, Tugwell P, Knottnerus A: GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. 2011, 64 (4): 380-382. 10.1016/j.jclinepi.2010.09.011.
Hemens BJ, Haynes RB: McMaster Premium LiteratUre Service (PLUS) performed well for identifying new studies for updated Cochrane reviews. J Clin Epidemiol. 2012, 65 (1): 62-72. 10.1016/j.jclinepi.2011.02.010.
Shekelle PG, Newberry SJ, Wu H, Suttorp M, Motala A, Lim Y-W: Methods Research Report. AHRQ Publication No. 11-EHC042-EF. Identifying Signals for Updating Systematic Reviews: A Comparison of Two Methods. 2011, Rockville (MD): Agency for Healthcare Research and Quality, Available at http://effectivehealthcare.ahrq.gov/.
Shultz M, De Groote SL: MEDLINE SDI services: how do they compare?. J Med Libr Assoc. 2003, 91 (4): 460-467.
Moher D, Tsertsvadze A, Tricco A, Eccles M, Grimshaw J, Sampson M: Cochrane Database of Systematic Reviews 2008, Issue 1. Art. No.: MR000023. When and how to update systematic reviews. 2008
Tsertsvadze A, Maglione M, Chou R, Garritty C, Coleman C, Lux L: (Prepared by the University of Ottawa EPC, RAND Corporation–Southern California EPC, Oregon EPC, University of Connecticut EPC, RTI–University of North Carolina EPC, Johns Hopkins Bloomberg School of Public Health EPC under Contract No. 290-02-0021 EPC2). AHRQ Publication No. 11-EHC057-EF. Updating Comparative Effectiveness Reviews: Current Efforts in AHRQ’s Effective Health Care Program. Methods Guide for Comparative Effectiveness Reviews. 2011, Rockville, MD: Agency for Healthcare Research and Quality, Available at: http://www.effectivehealthcare.ahrq.gov/reports/final.cfm.
Acknowledgements
Members of the Updating Guidelines Working Group: Martínez García L, Arévalo-Rodríguez I, Solà I, Haynes RB, Vandvik PO, Alonso-Coello P, Díaz del Campo P, Estrada MD, García Álvarez EE, Gracia J, Kotzeva A, Salcedo-Fernandez F, Trujillo-Martín MM. Laura Martínez García is a doctoral candidate at the Pediatrics, Obstetrics and Gynecology, and Preventive Medicine Department, Universitat Autònoma de Barcelona, Barcelona, Spain.
Source of support
This study is partially funded by a grant research from the Instituto de Salud Carlos III (FIS PI10/00346) and within the framework of collaboration of the Quality Plan for the Spanish National Health System, under the terms of the collaboration agreement signed by the Carlos III Health Institute and the Aragon Health Science Institute, as technical secretariat GuiaSalud-Biblioteca project. Laura Martínez García is funded by a Río Hortega research contract from the Instituto de Salud Carlos III (CM11/00035). Pablo Alonso-Coello is funded by a Miguel Servet research contract from the Instituto de Salud Carlos III (CP09/00137).
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
Conceiving the review: LMG, PAC. Undertaking searches: IS, LMG. Screening search results: LMG, Ingrid IAR. Screening retrieved papers against inclusion criteria: LMG, IAR, IS. Extracting data from papers: LMG, IAR. Data management for the review: LMG. Writing the review: LMG, PAC. Comment and editing of review drafts: LMG, PAC, IAR, IS, R Brian Haynes, Per Olav Vandvik, Petra Díaz del Campo Fontecha, Maria Dolors Estrada Sabadell, Elvira García Álvarez, Javier Gracia San Román, Anna Mariyanova Kotzeva, Flavia Salcedo Fernandez, María del Mar Trujillo Martín. Responsible for reading and checking review before submission: LMG, PAC. All authors read and approved the final manuscript.
Electronic supplementary material
13012_2012_540_MOESM1_ESM.doc
Additional file 1: Search strategy. This document shows the search strategy in MEDLINE and The Cochrane Methodology Register. (DOC 27 KB)
13012_2012_540_MOESM2_ESM.doc
Additional file 2: Excluded studies. This document shows a list with excluded studies and the reason for exclusion. (DOC 66 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Martínez García, L., Arévalo-Rodríguez, I., Solà, I. et al. Strategies for monitoring and updating clinical practice guidelines: a systematic review. Implementation Sci 7, 109 (2012). https://doi.org/10.1186/1748-5908-7-109
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1748-5908-7-109